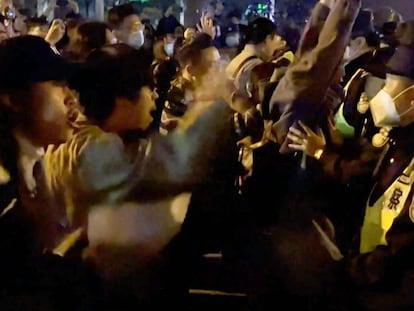Aseptic meningitis outbreak raises alarm in Mexico
Eleven young women and one man have died so far and there have been more than 60 infections reported after a fungus was found in several batches of anesthetic


The northwestern Mexico state of Durango is on alert after an outbreak of aseptic meningitis. At least 12 people – 11 women and one man – have died during the past month in four private state hospitals due to a fungus present in four batches of bupivacaine, a local anesthetic. There have been more than 60 confirmed positive cases reported, the vast majority of them young women, according to the Durango State Health Secretariat (SSD). Despite the fact that the authorities have set up special areas in two public health centers to treat the infection, the situation has not yet stabilized and the number of cases is continuing to rise slowly.
“It will easily reach about 100 infections. The fungus is very aggressive in spite of treatment. Mortality can exceed up to 50% of cases. It is a tragedy; it will go down in the history of medicine”, says Eder Zamarrón, an intensive care specialist at the Hospital MAC Tampico. Experts concur the most probable hypothesis is the bupivacaine was contaminated by mishandling at health centers, although no one has so far assumed responsibility and no arrests have been made. “It would be speculating to attribute the cases to the bottles used or stored or applied as anesthetics to patients,” Mexico’s under-secretary for health, Hugo López-Gatell, said in a statement.
The illness is not contagious but there is no record of how many patients received the anesthetic and may have been exposed to the fungus. The health authorities are monitoring everybody who may have been given bupivacaine since March in any of the four affected hospitals – Parque, Santé, Dikcava and San Carlos, all located in the city of Durango and now temporarily closed – a number that could be in the hundreds. EL PAÍS has requested an interview with the SSD but at the time of this article going to print no response has been received. “The investigation to determine the causes of the meningitis will take time and will be focused on determining at what point in the process the contamination occurred,” López-Gatell Ramírez said in his statement, which added another 12 women are currently in serious condition.
The alarm in Durango over meningitis – a disease that inflames the tissues that cover the brain and spinal cord – was raised in early November. At that time, the origin of the disease was still unclear. Although it is normally transmitted by direct contact with an infected person, medical tests revealed that in Durango’s case the cause was a fungus - Fusarium solani - present in batches of bupivacaine and heavy bupivacaine. The drug is a local anesthetic used for short surgeries, its effects usually lasting around two hours. The contaminated batches were manufactured by Pisa, a pharmaceutical company based in Mexico, although the experts consulted agree that the fungus had been present in the drug since it was produced, stating it was most likely compromised in the four hospitals where the infections have been recorded, as Pisa supplies bupivacaine across Mexico and to some hospitals in other Latin American countries.
“To save resources they have reused the needles”
Consuelo Tinajero, an anesthesiologist at Silao General Hospital in Guanajuato, has been closely following the outbreak: “As anesthesiologists we were notified that cases of meningitis were occurring in the city of Durango and these were being associated with the use of bupivacaine. We were notified of the specific batches that were used and we were warned of the risk that existed if we gave an anesthesia. What strikes me is that Pisa distributes to the whole country but nowhere else have these types of cases been reported. At the hospital where I work, we perform a minimum of 12 to 15 neuraxial anesthesia procedures daily because we work with many pregnant patients and for a cesarean section, it is the ideal anesthesia.”
The vast majority of infected patients are young women who have given birth in the last few months and underwent caesarean sections. Alejandro Macias, one of the leading infection specialists in Mexico, says: “It is used less in men, for example, in hemorrhoid operations, which explains why basically women who have had cesarean sections are affected. We can practically rule out the possibility that the medication is contaminated, unless it was contaminated at the site of use. One of the most probable hypotheses is that, in order to save resources, the needles were reused.”
The needles, Macias explains, are disposable and inserted into the syringe through plastic, which cannot be disinfected at high temperatures because the material melts. “There’s a thing called cold sterilization, but it’s based on the false idea that you can sterilize something without using an autoclave [a high-temperature sterilizing machine]. They often use disinfectants and then cold disinfection systems thinking that it’s going to leave the needle sterile but it’s not true; those systems don’t sterilize the inside of the needle. If it was contaminated and the fungus was present inside it, they would have injected the analgesic into the central nervous system.”
Tinajero agrees, explaining that in the type of anesthesia for which bupivacaine is used, the drug is introduced directly into the central nervous system using a “very thin” needle. “I suspect the syringes: they used to be made of glass, they were not discarded but re-sterilized, sometimes with heat or with other substances, including alcohol. I think these hospitals are still using this type of reusable glass syringe.”
The experts consulted also reject the idea that, despite most of the affected patients being women, what has happened in Durango is a case of obstetric violence - “violence exercised by health professionals towards pregnant women during childbirth and the postpartum period”, as defined by the World Health Organization. “I would not label it as obstetric violence. I think the doctors who administered the anesthesia acted correctly and did not know the risk they were subjecting the patients to,” says Tinajero.
“The responsibility lies with the hospital managers”
“Another hypothesis,” Macias points out, “is that they may have bought the medicine on the black market, where they sometimes prepare the drugs in a garage. If they bought it there to save resources, labels may have been falsified or complete batches contaminated.” Does that constitute medical negligence? “Absolutely, yes. It’s a problem that happens a lot in developing countries to save resources. If that is the case, this is one of the worst instances of failures in the most basic measures of care I have ever seen.”
Tinajero, however, stresses: “I would not call it medical negligence because we as physicians and anesthesiologists have no way of knowing if the medication is contaminated or if the needles have been sterilized, although we can tell if they give us reusable syringes, if they are made of glass and not plastic. The responsibility lies with the hospital directors; they know that they are not sterilizing properly, or are re-sterilizing single-use material, or if they bought apocryphal medication. The hospital’s supplier should be aware of that, and they should be investigated.”
Sign up for our weekly newsletter to get more English-language news coverage from EL PAÍS USA Edition